Preimplantation genetic testing (PGT)
Preimplantation genetic testing (PGT) allows the examination of embryos created through in vitro fertilisation (IVF) before they are transferred to the uterus. The goal is to detect genetic abnormalities that often lead to early miscarriages and ensure that only healthy embryos with a higher chance of successful pregnancy are transferred to the uterus. There are three main types of PGT – PGT-A, PGT-SR, and PGT-M. This procedure also allows for HLA typing to facilitate the birth of a child who may help in the treatment of a sick sibling.
Who is PGT suitable for?
Who is PGT suitable for?
Preimplantation genetic testing (PGT) can be useful if you are concerned about hereditary diseases or pregnancy complications. The testing helps increase the chance of having a healthy child by detecting genetic issues before the embryo is transferred to the uterus. Consider PGT if:
- You or your partner have a genetic mutation that you would like to avoid passing on to your future child.
- One of you has chromosomal abnormalities that could lead to miscarriages or health problems in the child.
- Your previous pregnancy was affected by genetic abnormalities.
- You are over 35 years old and want to reduce the risk of genetic defects, such as Down syndrome.
- You have experienced recurrent miscarriages or multiple unsuccessful IVF attempts.
- Your partner has sperm quality issues, and you plan to use the ICSI method.
- One of you has recently undergone cancer treatment.
PGT can also help if there is a risk of hereditary diseases in the family, such as cystic fibrosis or haemophilia. Thanks to PGT, it is possible to select an embryo that is not affected by the disease or that may be a suitable donor for a sick sibling.
Our goal is to help you feel more confident and have a better chance of a healthy pregnancy. We are here to assist you through this process with peace of mind and understanding.

How does PGT work?

How does PGT work?
Preimplantation genetic testing (PGT) is a procedure that significantly increases the chances of a healthy pregnancy. During this process, called a biopsy, we take 5–10 cells from the embryo. This procedure is performed by an experienced embryologist on the fifth or sixth day after fertilisation.
- Day 5/6: At the blastocyst stage of the embryo, we collect 5–10 cells for examination, and immediately after the collection, the embryo is frozen. The test results are usually available within 2–4 weeks, so the embryo is transferred to the uterus in one of the following cycles.
This process begins with a genetic consultation, where it is decided whether to focus on hereditary diseases or genetic changes caused by age or environmental factors. Embryo biopsies are carefully planned to help achieve the goal of a healthy baby.
What are the types of PGT and when to choose them?
PGT-A: Preimplantation Genetic Testing for Aneuploidy
PGT-A is a diagnostic test that focuses on detecting chromosomal abnormalities, known as aneuploidy, in embryos before their transfer to the uterus. Aneuploidy means that the embryo has an incorrect number of chromosomes, which can lead to failed implantation, early miscarriages, or the birth of a child with a genetic defect, such as Down syndrome.
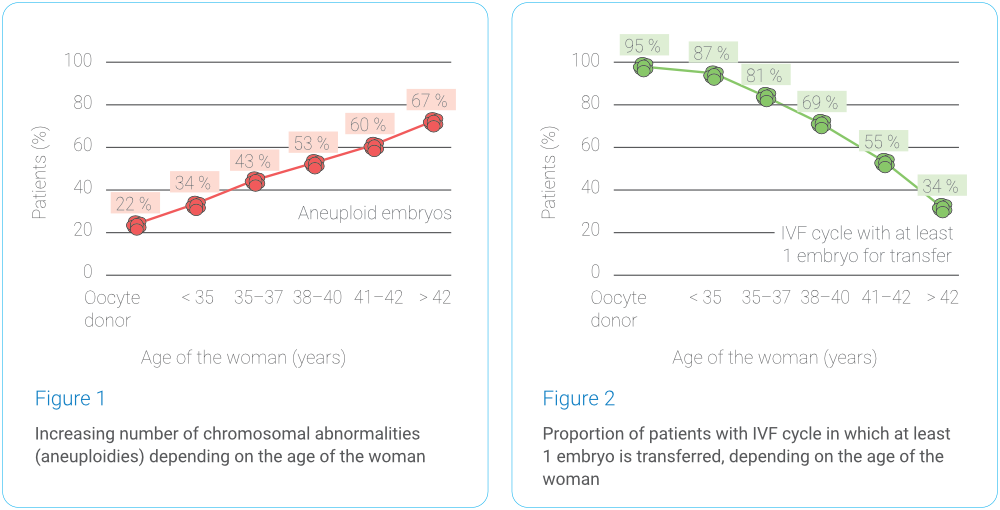
Chromosomal abnormalities do not spare even young women – an incorrect number of chromosomes can be detected in approximately one in five embryos in younger women. Just before menopause, aneuploidy is present in almost every egg. The risk of aneuploidy increases significantly around the age of 35, when the chances of finding an embryo with the correct number of chromosomes drop rapidly. The probability of finding an embryo with the correct chromosomal makeup in different age groups is illustrated in Graphs 1 and 2 (data analysis by Gennet Ltd., 3,347 IVF cycles, 10,307 embryos examined).
Who is PGT-A suitable for?
PGT-A is recommended primarily for those who have an increased risk of chromosomal abnormalities, such as:
- Women over 35
- Couples with recurrent pregnancy losses
- Couples with unsuccessful IVF cycles
- Couples who have already had a child with a chromosomal abnormality or a miscarriage due to a chromosomal abnormality
- Men with severe sperm production disorders
- Those who have undergone chemotherapy or radiotherapy
During an IVF cycle, embryos are cultured until day 5 or 6, when they reach the blastocyst stage. At this stage, 5–10 cells are taken from the embryo, which is then frozen. Test results are available within 2 weeks. If the embryo is healthy, it can be transferred to the uterus in the next cycle.
PGT-A helps increase the chance of a successful pregnancy by allowing the transfer of only healthy embryos without chromosomal abnormalities. This reduces the risk of miscarriages and shortens the time needed to achieve a successful pregnancy and the birth of a healthy baby.
What do the test results look like?
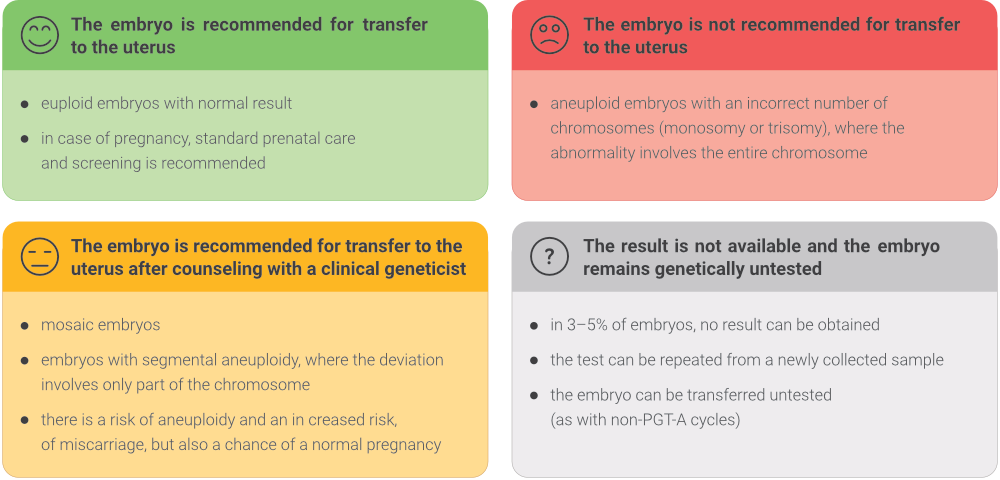
The examined embryos can be divided into 4 basic groups based on the examination conclusions and recommendations for further action:
What are the limitations of PGT-A and its reliability?
- The biopsy of the embryo on the 5th or 6th day of development at the blastocyst stage does not pose any risk to the foetus or the newborn according to current knowledge. It also does not reduce the chance of the embryo surviving freezing or successfully implanting in the uterus.
- In a small percentage (3–5%) of cases, it may be difficult to obtain a result from genetic testing due to handling a small sample. In these cases, the embryo can be thawed again, and the testing can be repeated, or a decision can be made to transfer the untested embryo.
- PGT-A can determine suitable embryos for transfer with more than 97% accuracy, but it cannot detect defects caused by random mutations or developmental abnormalities without a clear genetic cause, which can also occur in natural pregnancies (3–5%). Therefore, the same prenatal care is recommended for pregnancies after PGT-A as for other pregnancies.
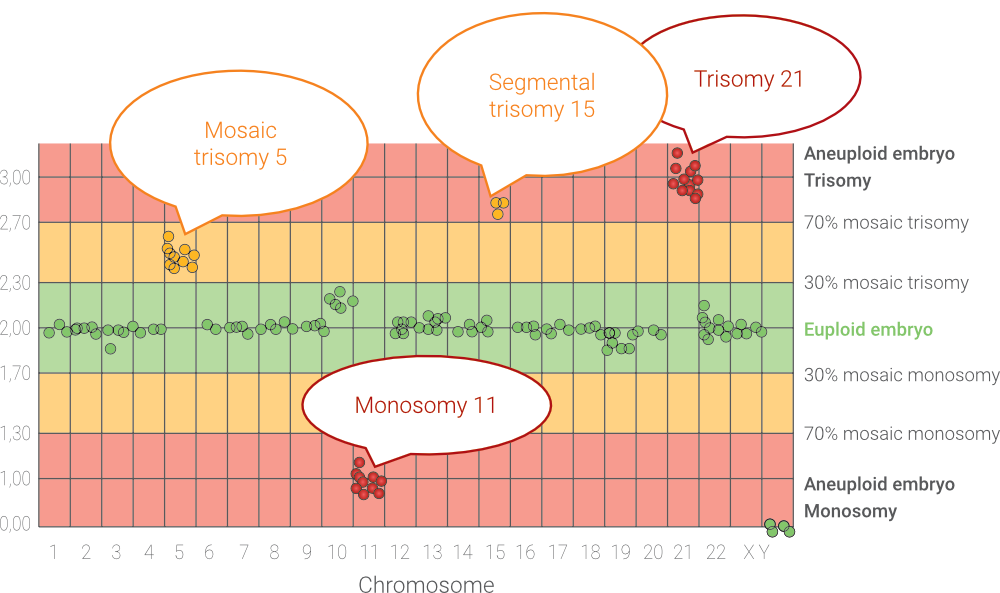
- Sometimes, the biopsied trophoblast cells may only partially reflect the genetic makeup of the embryo. Genetic changes may appear in 5-10% of embryos, which typically disappear during development. Nevertheless, these abnormalities may be associated with certain risks, but the embryo still has a chance of a successful pregnancy and healthy birth. The transfer of such an embryo is possible after consultation with an IVF specialist and a clinical geneticist.
- If there are few embryos at the blastocyst stage available, it may happen that all have a chromosomal abnormality. PGT-A can reveal this fact, but it is not its cause, nor can it eliminate it.
PGT-M: Preimplantation Genetic Testing for Monogenic Disorders and PGT-SR: Preimplantation Genetic Testing for Structural Rearrangements
PGT-M: Preimplantation Genetic Testing for Monogenic Disorders and PGT-SR: Preimplantation Genetic Testing for Structural Rearrangements
PGT-M and PGT-SR are tests focused on specific genetic issues. PGT-M is used to test embryos for known monogenic diseases (diseases caused by damage to a single gene), such as cystic fibrosis or haemophilia, while PGT-SR is intended for couples where one or both partners have a known chromosomal rearrangement that may lead to serious health problems in the child.
Who is PGT-M / PGT-SR suitable for?
- You or your partner are carriers of a mutation or have a genetic disease or chromosomal rearrangement.
- You have had to terminate a pregnancy due to a genetic disease or chromosomal changes in the foetus.
- You have a child with a serious genetic disease or chromosomal defect.
This type of testing allows for a reduced risk of having a child with a serious genetic disorder or chromosomal defect and helps ensure that only healthy embryos are transferred.
PGT is an important tool in planning a safe and healthy pregnancy and is always performed based on careful consultation with a geneticist.
What does PGT involve?
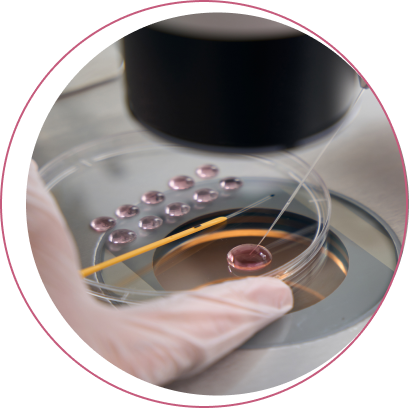
What does PGT involve?
All types of preimplantation genetic testing (PGT) require an in vitro fertilisation (IVF) cycle to genetically examine the obtained embryos. The entire process begins after an initial consultation with an IVF physician and the start of menstruation.
After 12–15 days of controlled ovarian stimulation, the egg retrieval follows. Once the eggs are fertilised with sperm, they are cultured until they reach the blastocyst stage, typically 5 or 6 days after retrieval.
At this stage, a biopsy is performed, during which several cells are taken from each embryo for genetic testing. These biopsied embryos are subsequently frozen (vitrified) and await the results of genetic testing. The results are available depending on the type of test performed.
Through testing, we determine which embryos are chromosomally normal and free of genetic defects. Subsequently, the patient’s uterus is prepared for embryo transfer. Suitable embryos are thawed and transferred into the uterus, while the remaining embryos are preserved for potential future use.
What are the advantages of PGT?
The goal of preimplantation genetic testing (PGT-A) is to facilitate the path to having a healthy child by excluding embryos with chromosomal abnormalities before transferring them to the uterus.
Transferring embryos with genetic defects often results in unsuccessful pregnancies and can lead to miscarriages. If a miscarriage occurs, another attempt to transfer an embryo may be delayed for several months. While PGT-A does not guarantee pregnancy, it significantly increases the chance of success by:
- Reducing the number of unsuccessful transfers, thereby lowering psychological stress.
- Decreasing the risk of spontaneous miscarriages.
- Lowering the risk of pregnancies with embryos that may have chromosomal abnormalities.
- Helping to avoid unnecessary embryo transfers, which saves time, costs and speeds up the path to having a healthy child.
- Provides important information that can help you make decisions about your treatment options, such as choosing donated eggs, sperm, or embryos.
PGT-A is a step that can bring peace of mind and support you on your journey to parenthood.
How much does PGT cost?
How much does PGT cost?
If, after consultation with a clinical geneticist, it is determined that there is an increased risk of chromosomal abnormalities in the embryo, PGT-A may be covered by health insurance.
In situations where PGT-A is not covered by insurance, we can offer it for direct payment. The price is based on our clinic's price list - details will be provided upon request.

Frequently Asked Questions
We are able to test for all monogenic disorders, including:
- Huntington's disease
- Cystic fibrosis
- Thalassemia
- Duchenne muscular dystrophy
- Fragile X syndrome
- BRCA1/BRCA2 (hereditary breast/ovarian cancer)
Some genetic conditions affect only one sex, such as haemophilia and Duchenne muscular dystrophy. If it is not possible to accurately detect the genetic defect on the sex chromosome (mainly X) that causes the disease, PGT can be used to determine the sex of the embryos. Only embryos of the sex where the condition does not manifest and that have the correct number of chromosomes are transferred.
Sex selection is permitted only for medical reasons that specifically affect the particular sex of the child.
Current research shows that the likelihood of implantation of a biopsied embryo is the same as that of an embryo without a biopsy. The removal of several cells from the embryo for PGT purposes is completely safe, and children born after this process are healthy.
As part of preimplantation genetic testing, the woman undergoes a standard IVF cycle. The embryo is then vitrified and subsequently tested. We test for:
- The number of chromosomes or parts of chromosomes
- In the case of PGT-M, we test the genotype of the embryo (or predisposition to hereditary diseases, susceptibility to genetic disorders, risk of genetic disease, etc.)
During each genetic consultation for a couple planning IVF, it is essential to assess whether the criteria for PGT/PGT-A are met. A recommendation from a clinical geneticist and written consent from both partners is always required to perform genetic testing on embryos, as mandated by law.
If a clinical geneticist indicates PGT/PGT-A testing based on the criteria outlined in the Recommendations of the Czech Society of Medical Genetics and Genomics (ČLS JEP), this testing is covered by health insurance. This also applies in cases where the IVF cycle itself is not covered by health insurance (e.g., for women over 39 years old or couples who have exhausted their covered cycles). Couples only pay for certain micromanipulation techniques within IVF that are not covered by health insurance, such as advanced methods of fertilising eggs - like ICSI, PICSI, or extended embryo culture.
If a couple does not meet the criteria for PGT/PGT-A coverage by health insurance, a clinical geneticist may still indicate the testing, but the couple will have to pay the full price according to the clinic's price list.
GENNET Praha 1
Na Poříčí 26
110 00 Praha 1